The Group is furthering its contributions to the medical and research fields with its GeneSilicon® DNA chip incorporating special surface treatment technology to apply diamond-like carbon (DLC) coating to silicon substrates.
We have started sales of a new SF3B1 genetic mutation test kit related to myelodysplastic syndromes (MDS).
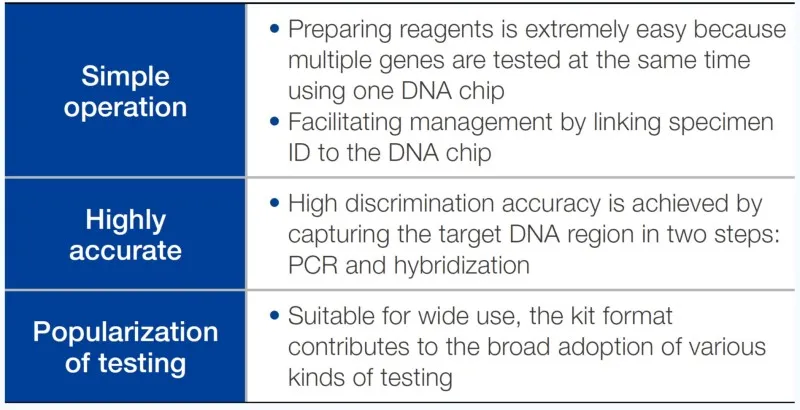
Through development and adoption of genetic mutation test kits that respond to a variety of ailments, we have been improving the quality of medical care and contributing to the realization of a better healthcare environment.
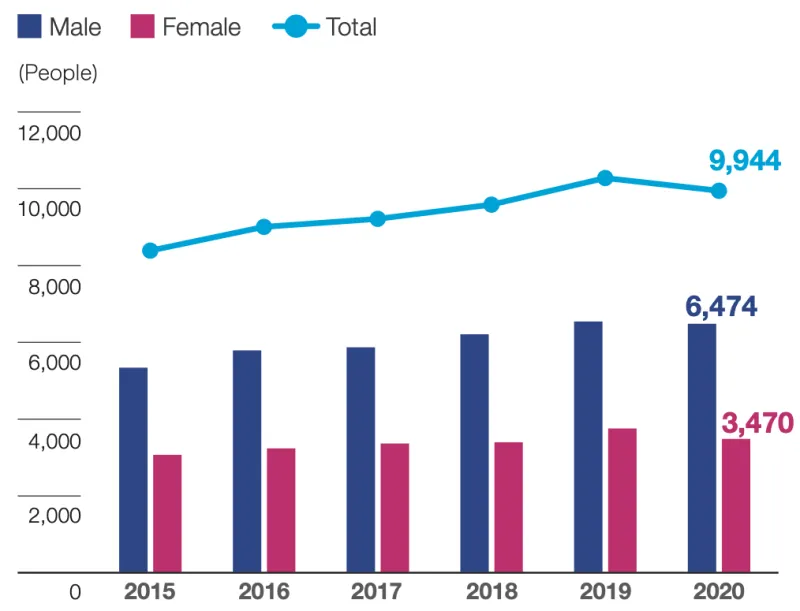
Toyo Kohan Co., Ltd. has been engaged since 2004 in the manufacture and sale of GeneSilicon® DNA chips incorporating its proprietary surface treatment technology. Equipped with functions where multiple genes can be analyzed at the same time using a single chip, these devices will contribute to the healthcare field in chronic disease diagnosis and can also be applied to investigations and research in the food industry. In 2020, we started selling DNA chips related to myeloproliferative neoplasm (MPN) and have now supported diagnosis and treatment decisions for over 10,000 patients.
In January 2023, we started sales of a new SF3B1 genetic mutation test kit related to MDS. Many MDS patients are elderly, with 10,000 cases (people) diagnosed each year in Japan. Genetic abnormalities clearly occur in the cells of some MDS patients, and the SF3B1 gene is one applicable gene.
These kits are currently used in contract analysis by major Japanese clinical testing labs. We will continue to pursue advancements in gene analysis technology, contributing to the progress of medical treatment and taking on the challenge of improving quality of life.
The MDS diagnosis criteria (WHO classification) specifies the SF3B1 genetic mutation as a major genetic abnormality. However, no company in Japan is accepting outside orders for this test, and so hope is being placed on making the testing possible at clinical sites. It has been suggested further that the test predicts response to novel therapeutic agents based on this
genetic variant, which makes the test recognized as an important one. Successful participation in the development of this genetic test kit has deep significance, and in the future, I would like to see progress on useful research for everyday clinics.